Everybody seems to want to climb on the bandwagon requiring the U.S. Food and Drugs Administration’s (FDA) to speed up its reviews, especially of drugs. Indeed, late last year, Republican presidential hopeful Senator Ted Cruz helped introduce the Reciprocity Ensures Streamlined Use of Lifesaving Treatments (RESULT) Act to do just this.
In early-July 2015, the House had overwhelmingly passed the 21st Century Cures Act which, amongst many other things, tried to do exactly the same. The proposed legislation was knocked on the head by Senate Committee on Health, Education, Labor and Pensions, when it said that, rather than take the act up, it would vote on a number of other bipartisan bills instead.
While there has been some evidence of some improvement within the FDA, delays can still lead both to lives lost and, for drug companies, because of the limits on patent times, revenues foregone. Actually speeding up reviews further, though, remains a knotty problem.
CDER New Molecular Entity (NME) and New Biologic License Application (BLA) Filings and Approvals
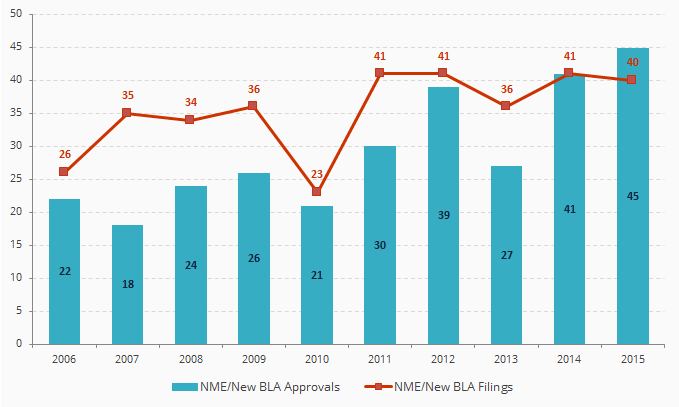
Source: FDA
CDER = FDA’s Center for Drug Evaluation and Research
Get the latest news & insights from MarketVector
Get the newsletterRelated: